The UK Government has agreed to revoke the regulations brought in last December that would have imposed onerous licensing requirements on producers and users of solvent and eco-solvent inks containing two substances – gamma butyrolactone (GBL) and 1,4-butanediol (BDO) – that had been reclassified as Class B controlled substances. A public consultation will be held with a view to new legislation against illicit use of these substances coming into force in early 2023.
The Graphics & Print Media Alliance (GPMA) has confirmed that ministers have agreed to revoke the Misuse Of Drugs (Amendment) (England, Wales and Scotland) Regulations 2021 (‘the 2021 Regulations’) without replacing them immediately. This means that in England, Wales and Scotland, organisations in the print supply chain will not require a licence for substances containing GBL on 15 June 2022, when the 2021 Regulations had been due to come into force.
The decision follows extensive representations to the Home Office by the members of the GPMA together with the wider chemicals supply chain, including the Chemicals Business Association and the Alliance of Chemical Associations.
Charles Jarrold, chair of the GPMA and chief executive of the BPIF, commented:
‘We are relieved that the proposed legislation has been withdrawn, and that a full consultation will now be carried out to determine the best way forward. We were particularly encouraged by, and appreciative of, the support that the Department for Business Energy and Industrial Strategy gave us in this process, and the level of engagement from the Home Office. Not only did we coordinate through the GPMA across the entire print supply chain, but we worked closely with the wider chemicals supply chain throughout this process.’
Tom Bowtell, chair of the Alliance of Chemical Associations and chief executive of the British Coatings Federation added:
‘We welcome that Government has listened to the concerns of industry and agreed to redraft the required regulations. This creates space for proper consultation with businesses as new legislation is drafted over the coming months. We hope these discussions will lead to a more proportionate and effective law, one which delivers on the Government’s aims but which does not unduly penalise legitimate users of these substances across the chemical supply chain, nor in the manufacturing or other sectors.’
The Home Office will now conduct a public consultation on how best to achieve the intended outcome of preventing the sale of bogus industrial products intended for illicit use. Subject to consultation and ministerial agreement, the intention is to seek views on the introduction of a licensing requirement which may contain some exemptions. Existing applicants for a licence will be contacted by the Home Office’s Drugs and Firearms Licensing to explain the next steps in relation to their application, with a view to new legislation coming into force in March 2023.
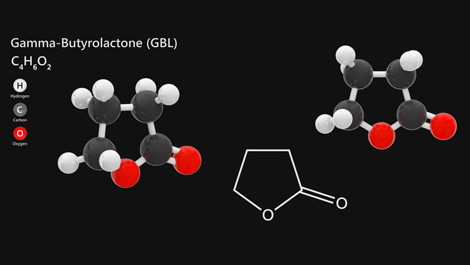

Regulation or not, awareness about chemicals used into inks and their potential effects on consumers is simply a corporate responsibility.
It is our pride to formulate free from concerning chemicals, law or not.
Indeed, Xavier, and more than one company potentially affected by this legislation has indicated its intention to move away from using these chemicals.